Common Beauty Product Injuries
Exploring Cosmetic Injuries Reported to the FDA From 2004 to 2018
2018 might have been the year for skin care innovation, but what if some trends weren’t all they were cracked up to be? Between 2004 and 2018, many people filed reports with the FDA about various cosmetic products users believe have caused adverse reactions – things like irritation, injury, and even cancer.

The last thing on someone’s mind when purchasing health and beauty products should be whether the product is safe. Cosmetic users likely take it for granted that something sold in stores and made available to the public will not cause harm. After analyzing over 14,000 cases of cosmetic products reported by users to the FDA from 2004 to 2018, we found this is definitely not always the case. Read on to see which beauty products reportedly cause the most adverse reactions.
Most Reported Beauty Products
In the sales and hospitality industries, consumer reviews and comments are extremely important. Word-of-mouth is one of the most significant ways for companies to find new business, which means they must be on high alert to ensure their clients and customers are satisfied with their products or services.
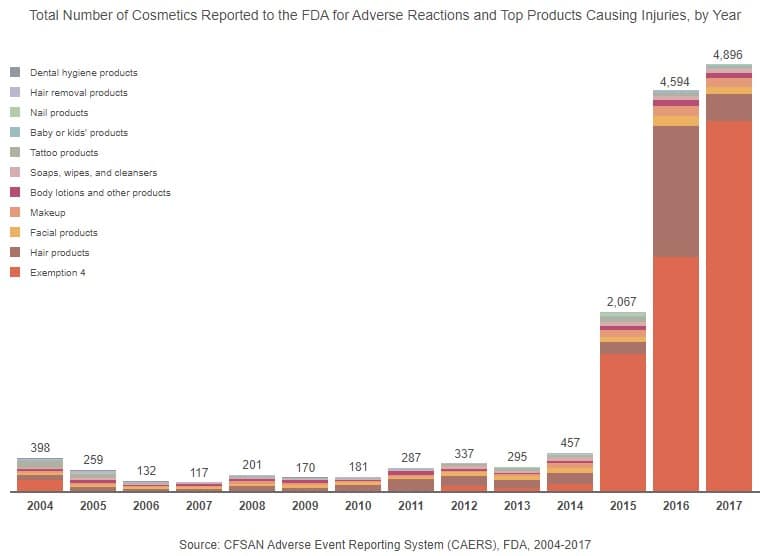
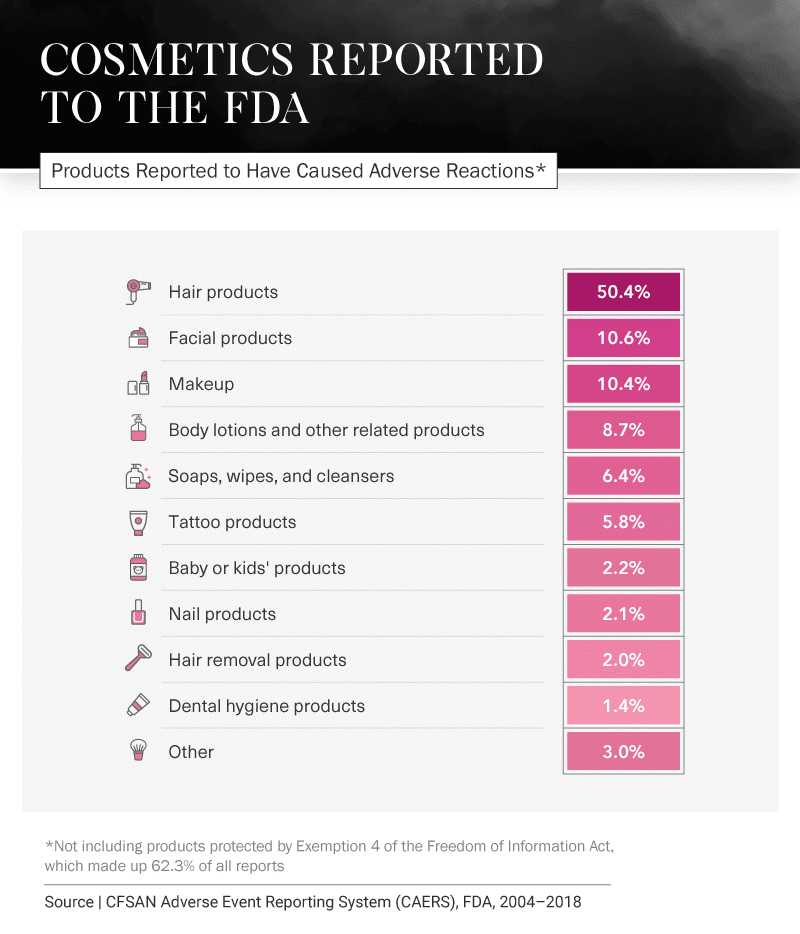
Still, it seems negative comments are often heard more loudly and clearly than those that are positive. Over the past 14 years, there have been many reports from consumers stating they have experienced injuries due to everything from hair products to toothpaste. Between 2004 and 2018, hair products had the most reports – not including reports that were hidden from the public due to Exemption 4 of the Freedom of Information Act (FOIA). The hair product category had the most reports by almost 40 percentage points.
Why so many adverse reports about cosmetic products? This could be because the industry is mostly self-regulated, which means there are no government regulations that determine which ingredients can be used. Companies are left to test and retest products and may find it’s not really necessary to go through so many intricate steps compared to medicine approval, for example. In fact, even a board-certified dermatologist from Northwestern University’s Feinberg School of Medicine in Chicago, Dr. Xu, emphasizes this point, calling the act of mandating a clinical study on every product available “somewhat impractical and somewhat ludicrous.”
Even though cosmetics and personal care products are not in the same category as medications, which require extensive amounts of testing and approval by the FDA, the outcomes can still be parallel. The government has no power over the regulation of beauty products unless consumers make their voices heard. The unfortunate part of this, however, is that even with multiple consumer complaints, a product can still be on the market for years until the government reaches a negotiation or the company decides to recall the item on its own. But it is still important for consumers to continue to raise their voice when something doesn’t seem right or causes an adverse reaction.
The Growth of Hidden Reports
Our analysis shows an emerging pattern in the products that are most reported each year. For example, hair products were often reported from 2005 to 2014. However, in 2015, there was a shift, and hair products became the second most reported product, while products covered by Exemption 4 of the FOIA were No. 1 and continued to be the most reported type of product through 2017.
Also, in 2015, there was a large increase in total reported adverse reactions. From 2004 to 2014, annual complaint totals never reached 500 (there were only 117 complaints in 2007). In 2015, however, reported injuries soared to a high of 2,067 and continued to increase, doubling in the following year. From 2014 to 2016, there were increasing numbers of reports filed about adverse effects caused by hair products. During the same time period, Monat, a shampoo and hair care line, faced lawsuits from consumers who claimed that the product caused hair loss, breakage, balding, itching, and rashes. Other hair product companies, such as WEN, faced class action lawsuits in 2015, as well.
Around the same time, there were also approximately 700 lawsuits against Johnson and Johnson, linking talcum powder use to ovarian cancer. Since then, the company has had to reinvent itself in its branding efforts in order to gain back the trust of consumers. The increase of ovarian cancer cases may have contributed to the growing number of reports filed under Exemption 4 during that time period.
The Cost of Beauty
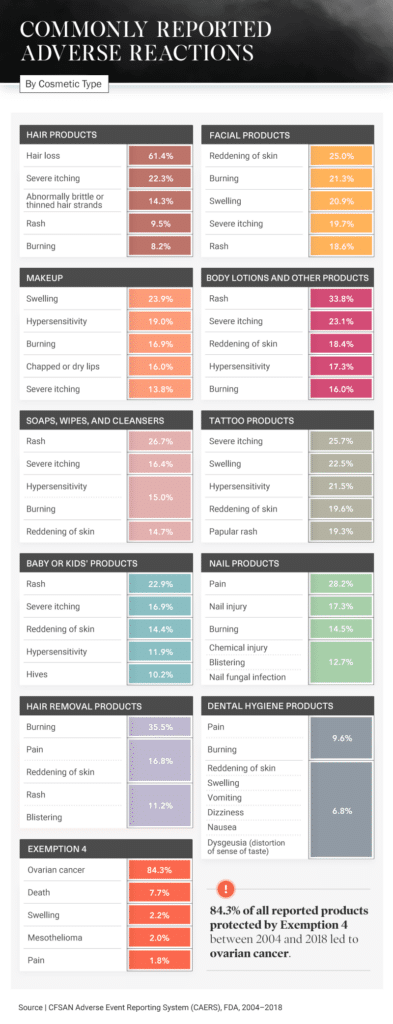
Unfortunately, our analysis shows that one of the most commonly reported side effects is also among the most severe. Out of all the product reports protected by Exemption 4, 84.3 percent showed that ovarian cancer was connected to the use of a particular cosmetic product.
Another side effect was hair loss, a complaint included in 61.4 percent of reports on hair products. Hair care companies can sometimes use marketing tactics and buzzword-filled taglines to attract consumers, saying their products are natural, organic, or nourishing, but even cosmetic companies that make these promises can fall short.
Additional consumer-reported side effects include reddening of the skin from hair removal products (16.8 percent), rashes from body lotions (33.8 percent), and pain from nail products (28.2 percent). Even baby and kids’ products, which are often marketed as safe and gentle, have been reported to cause rashes, severe itching, and hives.
A Terrifying Timeline
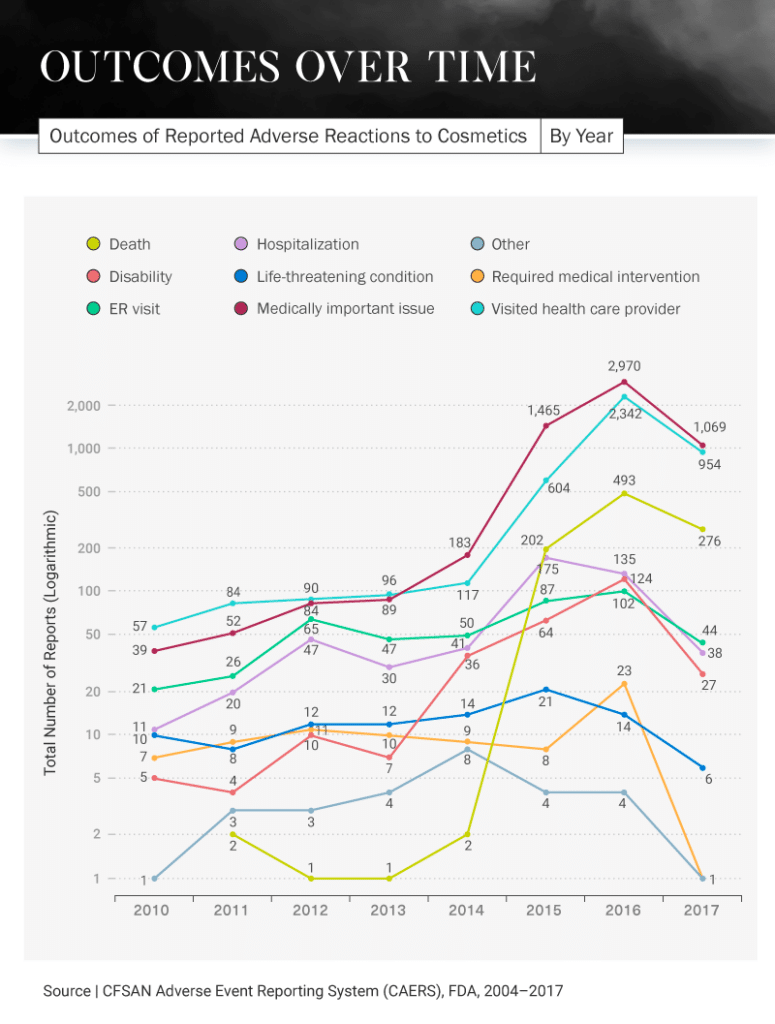
Not only are consumers reporting experiencing negative reactions to cosmetic products, but they are also experiencing severe outcomes in some cases. In 2016, there was a huge spike in the number of reported visits to health care providers because of cosmetic product side effects. More than 2,300 people reported going to their provider that year, compared to only 57 in 2010. Each year after that steadily increased, except for 2017 when there was a dip to 954.
What could have caused this intense increase in visits to the doctor? In 2016, there were 124 cosmetic product recalls – 117 of which involved some kind of chemical or microbiological issue. The most common issues stemming from these products were choking and burns, both of which would require a person to visit their health care provider.
Even more unfortunate news came in 2016: There was an increase in deaths caused by cosmetic products (493), compared to 202 in 2015 and just two in 2011. Thankfully, there was another decrease in 2017 for this category as well, dipping to 276. Hospitals saw the most patients because of cosmetic products in 2015 when 175 reported having to be hospitalized due to an adverse reaction, but this decreased to 135 in 2016 and 38 in 2017.
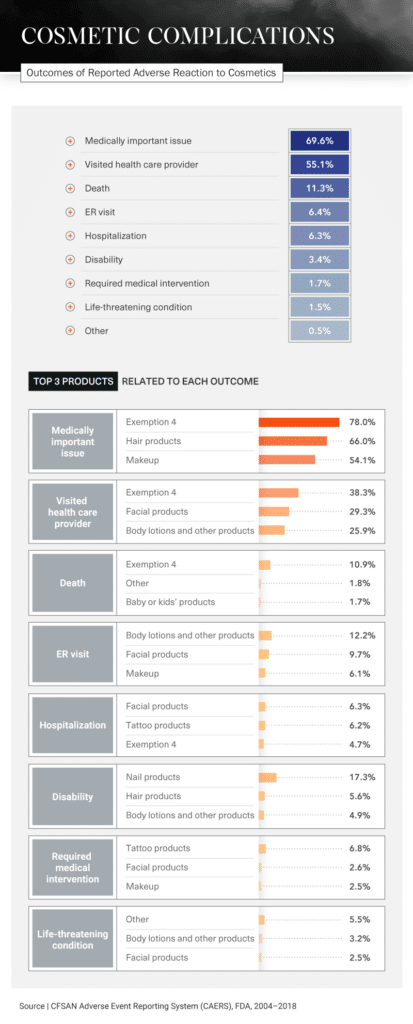
Medical Intervention
Different products cause different reactions, but many have had the same serious results: visits to the hospital or ER, disabilities, or in the most extreme situations, death. In fact, 11.3 percent of all incidents regarding cosmetic product side effects reported to the FDA between 2004 and 2018 ended with the person’s death. In more than half of all reports, the person involved had to visit a doctor.
Products that were most likely to require medical intervention included tattoo products, facial products, and makeup. Visits to a health care provider were typically caused by Exemption 4 products, facial products, and body lotions.
Of all reports involving nail products, 17.3 percent resulted in disabilities. Manicures and pedicures are often seen as self-care treats – a relaxing way to end the day. But what could start as a comfortable experience, massage chair and all, could end with an ER visit. Be wary of products used while getting your nails done, especially the pumice stone, which, if not used properly, could cause bacterial, fungal, or viral infections.
Legal Action May Be Needed
Take matters into your own hands when it comes to what you put on your body. As a consumer, it is your responsibility to read labels, do research on any ingredients you may not know about, and be aware of whether you have any skin conditions or allergies.
In the case that you do come face to face with an adverse reaction such as the ones we’ve talked about here, speak up for yourself. Take legal action if necessary – especially if you are one of the many women affected by the adverse effects of talcum powder, including ovarian cancer.
Call a law firm that you can trust to take your case seriously and help you reach justice. At Farah & Farah, our team of lawyers will advocate for you and build a strong case, negotiate your medical bills, and fight for a settlement or verdict that covers your expenses. We have your health and best interest in mind.
Methodology
For this analysis, we used public data sets from the CFSAN Adverse Event Reporting System (CAERS), published by the U.S. Food and Drug Administration. This is a post-marketing surveillance system that collects reports about adverse events involving a number of CFSAN/FDA-regulated consumer products. We looked at all 14,235 records about cosmetic products from 2014 to 2017 and categorized the reported products into broader groups:
Hair products
Shampoos, conditioners, gels, mousses, dyes, bleaches, perming and relaxing products, masks, and any other product intended for use in the hair. This did not include hair removal products.
Facial products
Facial moisturizers, lotions, serums, and anti-aging products
Body lotions and other body products
Body lotions, oils, tanning and skin bleaching products, perfumes, sprays, deodorants, and shaving and hair removal products
Soaps, cleansers, and wipes
Body wash, bar soaps, hand soaps, facial soaps, cleansing wipes, and makeup removers
Makeup
Eye shadows, eyeliners, mascara, blush, bronzer, highlighter, primers, lip products, foundations, powders, and concealers
Nail products
Nail polish, nail polish remover, gels, and tips
Baby and kids' products
Baby wash, baby powder, baby lotion and oil, and kids’ shampoos and soaps
To learn more about how each of the possible medical outcomes was defined, please visit the FDA website.
Limitations
According to the CFSAN, “The adverse event reports about a product and the total number of adverse event reports for that product in CAERS only reflect information AS REPORTED and do not represent any conclusion by the FDA about whether the product caused the adverse events. For any given report, there is no certainty that a suspected product caused a reaction.” Read more about the limitations and caveats about this data set here.
Although reported products may have actually caused an adverse event, the data do not specify if the consumer had any previous medical conditions or if they used the product improperly.
Additionally, we couldn’t classify every product, as some descriptions did not provide adequate information. Those were grouped in the “other” category.
Other products that were initially reported were redacted from the records based on Exemption 4 of the Freedom of Information Act. This protects trade secrets and commercial or financial information about companies. All of those products are labeled as “Exemption 4” in our visualizations.
Findings were not statistically tested.
Sources
- https://www.harpersbazaar.com/uk/beauty/beauty-shows-trends/a14415615/beauty-trends-2018/
- https://www.fda.gov/food/complianceenforcement/ucm494015.htm
- https://www.entrepreneur.com/encyclopedia/word-of-mouth-advertising
- https://www.justice.gov/sites/default/files/oip/legacy/2014/07/23/exemption4_0.pdf
- https://www.cbsnews.com/news/increasing-number-of-side-effects-from-cosmetics-study/
- https://www.nytimes.com/2017/08/07/well/for-cosmetics-let-the-buyer-beware.html
- https://www.abcactionnews.com/money/consumer/taking-action-for-you/hundreds-across-the-country-say-monat-shampoo-caused-balding-scalp-sores
- https://people.com/style/wen-hair-care-lawsuit-hair-loss-photos/
- https://theworld.org/stories/2015-05-24/women-are-suing-johnson-johnson-over-talcum-powder
- https://adage.com/article/cmo-strategy/johnson-s-baby-reinvents-kinder-gentler-simpler/314415
- https://www.womenshealthmag.com/beauty/a19430729/monat-lawsuit-hair-loss/
- https://www.refinery29.com/en-us/2018/03/193450/pedicure-infection-story
- https://www.fda.gov/food/compliance-enforcement-food/cfsan-adverse-event-reporting-system-caers
- https://www.fda.gov/media/97035/download
Fair Use Statement
Awareness is the first step toward finding solutions, so please feel free to share this study with anyone you know who may be interested in understanding more about possible outcomes of using cosmetics – as long as it’s for personal use. If you do, we simply ask that you give credit to this page with a link.